Treating Cancer from Within
Our Purpose
We create implantable devices to enhance brain tumour treatment and help more patients achieve long-term survival.
Introducting GRACE
A New Platform for Cancer Treatment
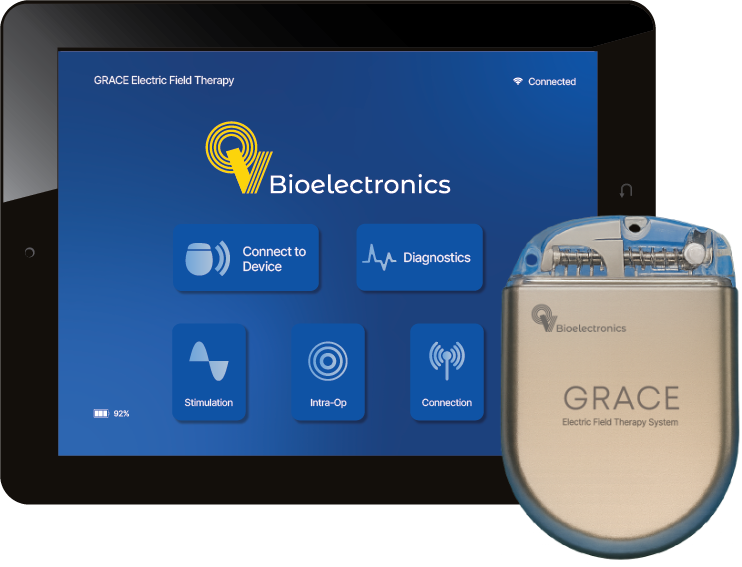
GRACE is an implantable device platform for treating solid tumours, starting with brain cancers. Placed at the time of surgery, it delivers therapy directly from within the body. Our initial focus is glioblastoma, the most common primary brain tumour in adults.

Why it Matters
Brain Cancer Treatment Hasn’t Kept Pace
Glioblastoma is among the most aggressive cancers. Current options are limited, survival rates remain low, and innovation is urgently needed. We are building technology to change that.
Diagnoses of Brain & CNS cancers globally every year
Deaths from Brain & CNS cancers globally every year
%
5-yr Survival for Brain Cancer
Our Technology
Harnessing the Power of Electric Fields
GRACE is designed to delivers continuous, precisely targeted electric fields to disrupt cancer cell division, killing cancer cells without harming healthy tissue. Its implantable design enables treatment to start sooner and work around the clock.
Contact Us
We are always open to connecting with clinicians, researchers, investors, and partners who share our vision for transforming cancer care. Whether you would like to learn more about our work, explore collaboration opportunities, or discuss investment, we would be delighted to hear from you.
General: [email protected]
Investors: [email protected]